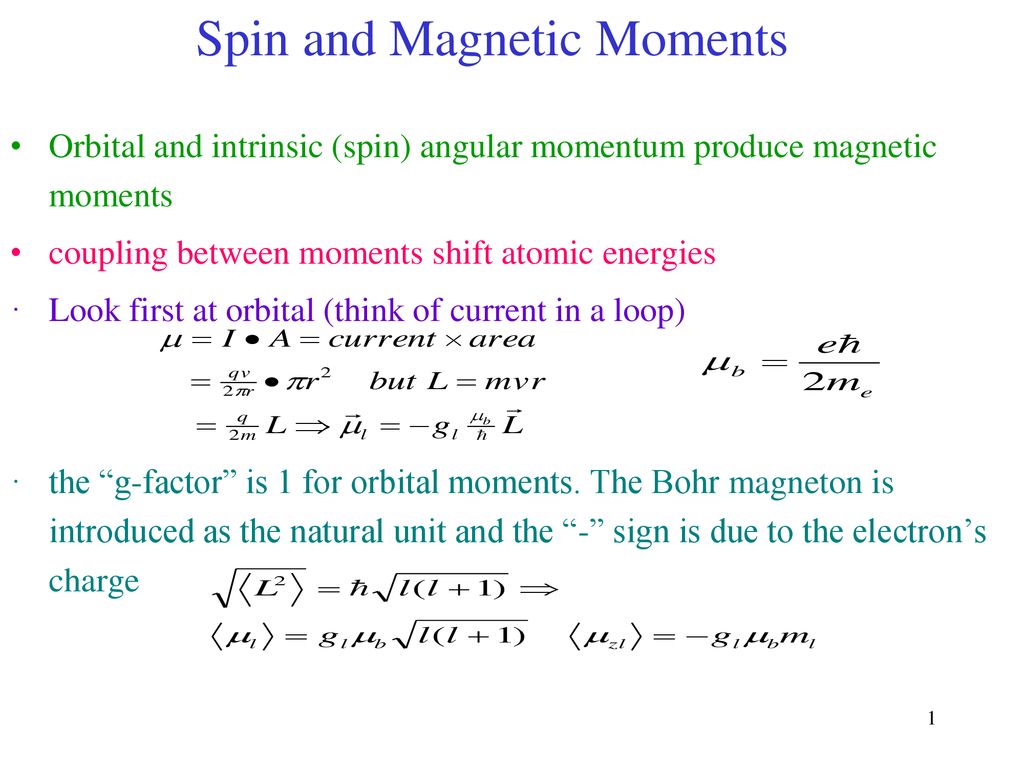
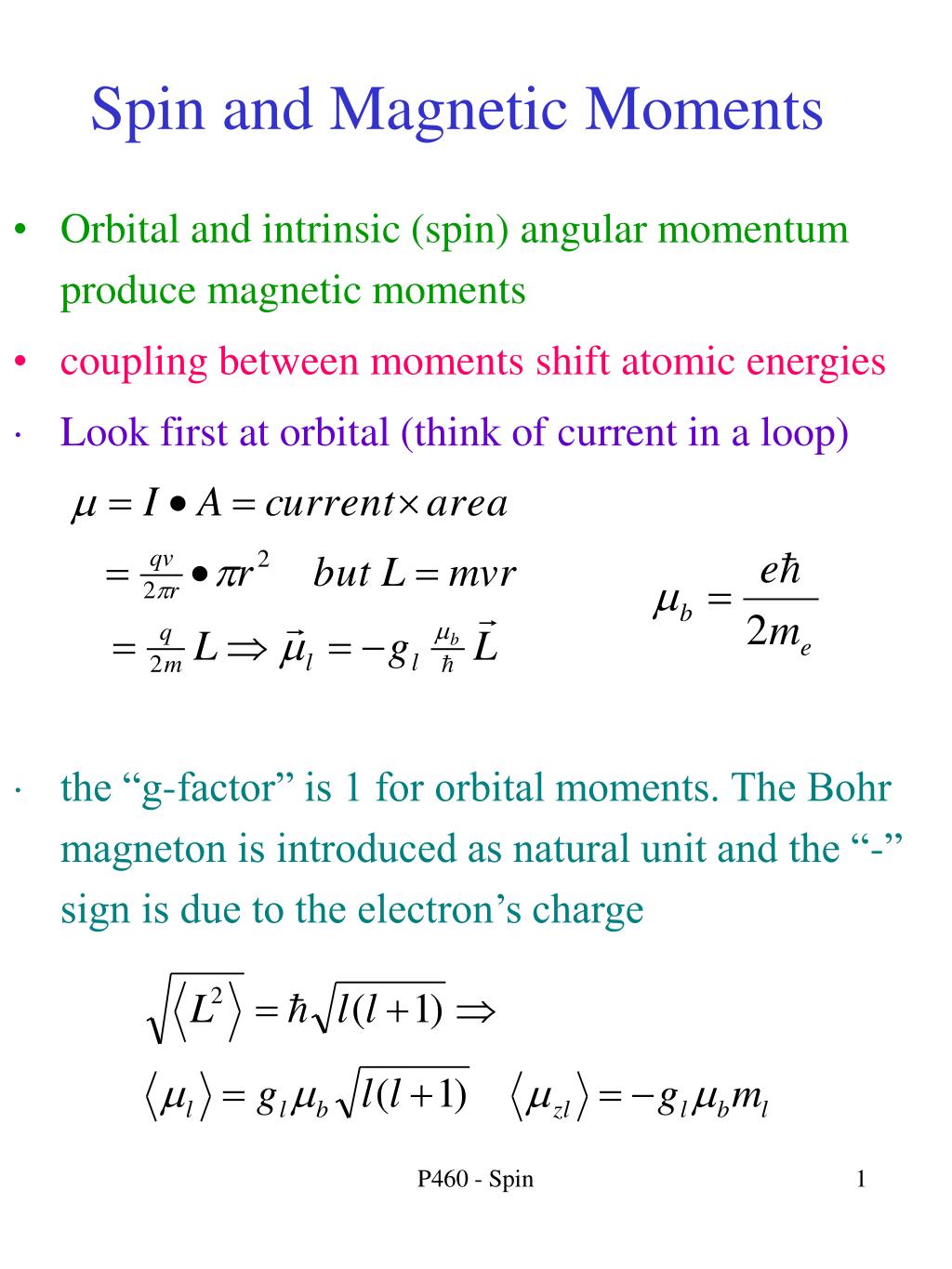
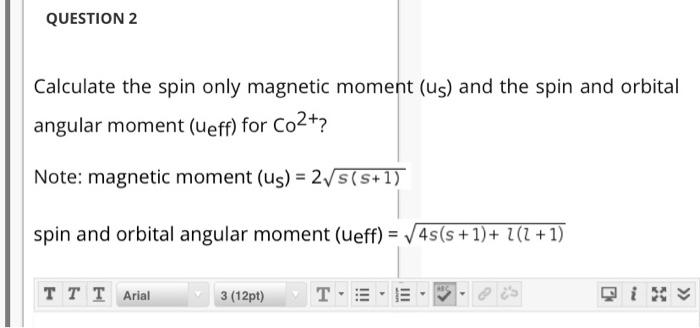
Write the spin only formula and give the unit of magnetic moment - Chemistry - Structure of Atom - 15663443 | Meritnation.com

The spin only magnetic moment of a divalent ion in aqueous solution (atomic number 29) is ______ BM. Option: 1 2 Option: 2 - Option: 3 - Option: 4 -

The highest value of the calculated spin-only magnetic moment (in BM) among all the transition - YouTube

Write the name of two metal which used in maximum composition in mischmetal. Calculate the value of the magnetic moment of V^{+2}

58. If spin only magnetic moment of vcl, is 1.73 BM then correct formula is :- * vci, (2) VCI, X (3) VCI, (4) VCI, 53 -1.732. 3ds socarz?

Q4)Calculate spin only magnetic moment of the following ions in aqueous state: (a) Mn2+ (b)Cr3+ (c)Co3+ - Chemistry - Aldehydes Ketones and Carboxylic Acids - 16918049 | Meritnation.com
What will be the theoretical value of spin only magnetic field when Fe(SCN)3 reacts with the solution containing F ions to yield a complex
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)


![ANSWERED] 140 The calculated spin only magnetic mom... - Physical Chemistry - Kunduz ANSWERED] 140 The calculated spin only magnetic mom... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20201001105030189875-2167239.jpg)